Is The Rusting Of Iron A Chemical Change
A metal is a fabric that has a sleeky appearance when freshly produced, polished, or shattered, and conducts electricity and heat reasonably well. Metals are either malleable or ductile (they may be hammered into sparse sheets) (tin be drawn into wires). Metals tin be chemical elements like iron, alloys like stainless steel, or molecular compounds like polymeric sulphur nitride.
The term "metal" is used more broadly in astrophysics to refer to all chemic elements in a star that are heavier than helium, rather than simply classical metals. In this sense, the first iv "metals" that accumulate in star cores through nucleosynthesis are carbon, nitrogen, oxygen, and neon, which are all chemically non-metals. Over the class of its being, a star fuses lighter elements, primarily hydrogen and helium, into heavier atoms. In this context, an astronomical object's metallicity refers to the proportion of its mass made up of heavier chemic elements.
Rusting of Iron
Rusting is the phenomenon of a reddish-brown coating forming on the surface of iron due to the action of wet air, and the cherry-brown coating is referred to as rust. Simply said, rust is a red-brown flaky substance that forms when an iron object is exposed to moisture air for an extended period of time. Rusting is the term for this phenomenon.
Rusting of iron and steel is the most prevalent case of metallic corrosion. Rusting of exhaust systems and vehicle bodywork, water pipes, and many sorts of structural steelwork are all well-known instances. The combined activity of air and water on atomic number 26 causes information technology to rust. Rusting does non happen in fully dry air or in the air that is completely devoid of water. Atmospheric weather and the relative contributions of the components that regulate rusting define the particular composition of the rust. It is primarily equanimous of hydrated ferric oxide, then the chemic formula of rust is Atomic number 26iiO3.xH2O .The following response can roughly characterise its formation:
4Fe + 3Oii +2xH2O → 2Fe2O3.xH2O
The outer surface of iron rusts beginning in the presence of wet air, and a layer of hydrated ferric oxide (rust) is deposited on the surface. This layer is fragile and porous, and if it becomes likewise thick, information technology may fall off. The lowest layers of fe are exposed to the environment, causing them to rust. Iron somewhen loses its strength as the process continues.
What is the process of Rusting of Iron?
Atomic number 26 rusting is an oxidation reaction. During rusting, iron combines with oxygen in the air in the presence of h2o to generate Fe2O3.xH2O, a hydrated iron (Iii) oxide.
This hydrated fe (Ill) oxide is referred to equally rust. Rust is largely hydrated fe (III) oxide, FetwoOiii.xH2O, every bit a result. The colour of rust is crimson-chocolate-brown. We've all noticed cherry-brown rust on iron nails, screws, pipes, and railings. When exposed to wet air, not just iron, only also steel, rusts. Steel, on the other hand, is more resistant to rust than iron.
Rusting of Iron is a Chemical Change
Rust is formed when iron (or an alloy of iron) is exposed to oxygen in the presence of moisture. This reaction is not instantaneous; rather, information technology takes place over a long period of fourth dimension. Iron oxides are formed when oxygen atoms combine with fe atoms. The bonds between the iron atoms in the object/structure are weakened as a upshot.
The oxidation state of iron increases equally a issue of the rusting reaction, which is followed by the loss of electrons. Rust is primarily composed of two types of iron oxides that differ in the oxidation land of the iron atom. These are the oxides:
- Iron (II) oxide is likewise known as ferrous oxide. This substance has an oxidation land of +ii and the chemical formula FeO.
- Iron(3) oxide, frequently known every bit ferric oxide, is a compound in which the iron atom has an oxidation land of +3. Fe2O3 is the chemical formula for this substance.
Iron is a reducing amanuensis, just oxygen is an splendid oxidising amanuensis. When exposed to oxygen, the iron atom hands gives away electrons. The chemical reaction is described equally follows:
Iron → Fe2+ + 2e–
When h2o is nowadays, the oxygen cantlet increases the oxidation land of iron.
4Feii+ + O2 → 4Fethree+ + 2O2-
The atomic number 26 cations and water molecules now undergo the following acrid-base reactions.
Fe2+ + 2H2O ⇌ Fe(OH)2 + 2H+
Iron3+ + 3HtwoO ⇌ Fe(OH)3 + 3H+
The direct reaction between the iron cations and the hydroxide ions besides produces atomic number 26 hydroxides.
O2 + H2O + 4e– → 4OH–
Fe2+ + 2OH– → Iron(OH)2
Fe3+ + 3OH– → Fe(OH)3
The iron hydroxides that result are now dehydrated, yielding the fe oxides that makeup rust. Many chemical processes are involved in this procedure, some of which are given below.
- Fe(OH)2 ⇌ FeO + H2O
- 4Fe(OH)2 + Oii + xH2O → 2Fe2O3.(x+4)H2O
- Fe(OH)3 ⇌ FeO(OH) + HtwoO
- FeO(OH) ⇌ Fe2O3 + HtwoO\
All of the chemical reactions listed above take i thing in common: they all require the presence of water and oxygen. As a result, the amount of oxygen and h2o surrounding the metal tin be limited to prevent rusting.
The Conditions Necessary for Rusting of Iron are:
Many factors contribute to the rusting of iron, including the amount of moisture in the air and the pH of the surrounding environment. The following are a few of these elements.
- Moisture: The availability of water in the environment limits the corrosion of atomic number 26. The virtually prevalent cause of rusting is exposure to rain.
- The rusting process is accelerated if the pH of the surroundings around the metal is depression. When iron is exposed to acid rain, it rusts more quickly. Atomic number 26 corrosion is slowed by a higher pH.
- Due to the presence of various salts in the water, fe rusts more quickly. Many ions in saltwater speed up the rusting process through electrochemical processes.
- Impurity: When compared to fe having a diversity of metals, pure atomic number 26 rusts more than slowly.
The size of the fe object tin can also influence how rapidly it rusts. A huge iron object, for example, is probable to have minor flaws due to the smelting process. These flaws provide a platform for environmental attacks on the metallic.
Experiment to Prove that Air and Moisture are Essential for Rusting:
Procedure to demonstrate that rusting requires moisture and air.
- Make clean atomic number 26 nails should be placed in each of the three exam jars labelled A, B, and C.
- Make full test tube A with tap water and cork it.
- Fill test tube B with hot distilled water, and then add roughly 1ml of oil and cork it. The oil will float on the surface of the water, keeping the air from evaporating.
- Fill test tube C with anhydrous calcium chloride and cork it. Whatsoever moisture in the air volition be captivated by anhydrous calcium chloride.
- Allow a few days for these test tubes to settle before observing.
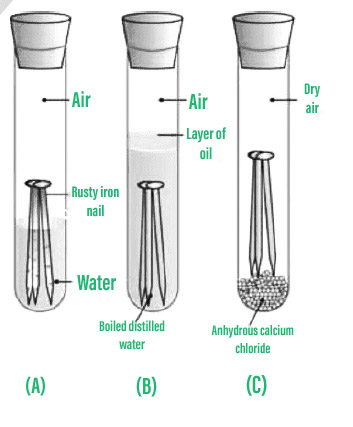
Observation: Iron nails rust in test tube A only not in test tubes B and C, according to the results. The nails in test tube A corroded because they were exposed to both air and water. Test tube B'south nails are solely exposed to h2o, but test tube C's nails are exposed to dry air.
Conclusion: This experiment demonstrates that rusting requires both air (oxygen) and wet to occur.
What are the damages caused past Rusting of Iron objects?
Rust is permeable and soft, and every bit it slips off the surface of a rusty iron object, the atomic number 26 beneath rusts. As a result, iron rust is a constant process that eats away at fe items over time, rendering them worthless. Rusting of iron causes pregnant impairment over time since it is used to build a wide range of structures and commodities, including bridges, grills, railings, gates, and the bodies of cars, buses, trucks, and ships. Information technology goes without saying that we should accept a way to keep atomic number 26 from rusting.
Prevention of Rusting of Iron
The loss of atomic number 26 objects due to rusting has a huge economical impact on the country, and information technology must be avoided. To keep atomic number 26 things from rusting, a variety of techniques are employed. To go along air and water out, the majority of the ways require covering the iron piece with something. The following are some of the most prevalent means to keep fe from rusting:
- Rusting of iron tin be prevented by painting: Coating the surface of the iron with paint is the most popular way to go along it from rusting. When the pigment is placed on the surface of an iron object, it prevents air and moisture from getting into impact with the object, preventing rusting. To prevent rusting, window grills, railings, fe bridges, steel effects, railway coaches, and the bodies of automobiles, buses, and trucks, among other things, are all painted on a regular footing.
- Rusting of iron tin be prevented by applying grease or oil: When grease or oil is placed on the surface of an iron object, air and wet are kept from coming into touch with information technology, preventing corrosion. Fe and steel tools and auto parts, for example, are rubbed with grease or oil to prevent corrosion.
- Rusting of fe can exist prevented by galvanisation: Galvanizing protects articles exposed to excessive wet, such as roof sheets and pipelines, against rusting. Galvanization is the technique of applying a thin layer of zinc to steel and iron to prevent rust. Galvanised iron is iron that has been zinc-coated. Zinc is more reactive than iron, therefore in the presence of moisture, it interacts with oxygen to generate an invisible layer of zinc oxide that protects it from further rusting. It's worth noting that fifty-fifty if the zinc blanket on galvanised iron products is broken, they remain rust-free. Because zinc is more reactive than fe, this is the case.
- Rusting of iron can be prevented past electroplating: Electroplating is another method for keeping items from rusting. In this procedure, noncorroding metals including tin, nickel, and chromium are electroplated on iron. This technique not simply keeps the goods from rusting simply besides improves their beauty. Bathroom fittings and vehicle elements such every bit cycle handlebars, motorcar bumpers, and then on are examples of chromium-plated items.
- Rusting of atomic number 26 can be prevented by alloying it to make stainless steel: Stainless steel is created when the fe is alloyed with chromium and nickel. Stainless steel is impervious to rust. Stainless steel cooking utensils, pair of scissors, and medical equipment, for example, do non corrode. Stainless steel, on the other mitt, is likewise expensive to be utilised in large quantities.
- Rusting of iron tin can be prevented by tinning: Tin is not-toxic, and its reactivity is lower than that of iron. Food cans are tinned, which implies that they have a thin layer of tin can on them. Every bit a event, when an electroplated thin coating of tin metallic is deposited on iron and steel items, the iron and steel objects are protected from rusting. Can-plated tiffin boxes are utilised because they are non-toxic and do not contaminate the food within.
- Rusting of iron can be prevented by Enameling: Enameling is a high-oestrus process that involves fusing powdered drinking glass into a metal substrate. Enamels can be used on a diversity of surfaces, including glass and ceramics.
Sample Issues
Question 1: What is the process of rusting atomic number 26?
Answer:
Iron rusting is an oxidation reaction. In the presence of water, the fe metal interacts with oxygen in the air to generate hydrated iron (III) oxide, Atomic number 26iiO3.xHiiO. This hydrated iron (Iii) oxide is referred to as rust. Rust is largely hydrated iron (Three) oxide, Fe2O3.xH2O, as a issue. Rust is a reddish-brown hue
Question 2: What is rusting of iron called?
Answer:
Rusting is the phenomena of a cerise-brown coating forming on the surface of iron due to the activity of wet air, and the carmine-chocolate-brown coating is referred to as rust.
Question 3: How rusting of iron can exist prevented?
Answer:
Rusting of iron can be prevented by
- Applying paint
- Applying grease or oil
- By galvanisation
- By electroplating
- Using alloying iron to make stainless steel
- By tinning
- Using Enameling
Question 4: What is rust? Requite the equation for the formation of rust?
Answer:
When fe is exposed to air for an extended period of time, it oxidises and develops a crimson-brown atomic number 26 oxide on the surface. Rust is the name for this reddish-brown textile.
Rust is formed via the following equation:
4Fe + 3O2 +2xH2O → 2FetwoO3.xH2O
Question v: How does rust impairment fe objects?
Respond:
Rust is permeable and soft, and as it slips off the surface of a rusty iron object, the fe below rusts. Equally a result, fe rust is a constant process that eats away at iron items over time, rendering them worthless. Rusting of iron causes significant damage over time since it is used to build a wide range of structures and commodities, including bridges, grills, railings, gates, and the bodies of cars, buses, trucks, and ships. Information technology goes without saying that nosotros should have a way to keep iron from rusting.
Question 6: What are the conditions necessary for rusting?
Answer:
Many factors contribute to the rusting of iron, including the amount of moisture in the air and the pH of the surrounding environment. The following are a few of these elements.
- Moisture: The availability of water in the surround limits the corrosion of iron. The most prevalent cause of rusting is exposure to pelting.
- The rusting process is accelerated if the pH of the environment effectually the metal is low. When fe is exposed to acrid rain, it rusts more rapidly. Iron corrosion is slowed by a college pH.
- Due to the presence of various salts in the water, iron rusts more chop-chop. Many ions in saltwater speed up the rusting process through electrochemical processes.
- Impurity: When compared to iron having a variety of metals, pure fe rusts more slowly.
Question 7: How does rust of iron be a chemic change?
Answer:
Rust is made up of iron oxide (Fe2O3). Every bit a result, rust and iron are non synonymous. Rust isn't the same affair as the atomic number 26 it's deposited on. Because a new component termed "iron oxide" is created during the rusting of fe, it represents a chemical change.
Source: https://www.geeksforgeeks.org/rusting-of-iron-explanation-chemical-reaction-prevention/
Posted by: buttshandeall.blogspot.com

0 Response to "Is The Rusting Of Iron A Chemical Change"
Post a Comment